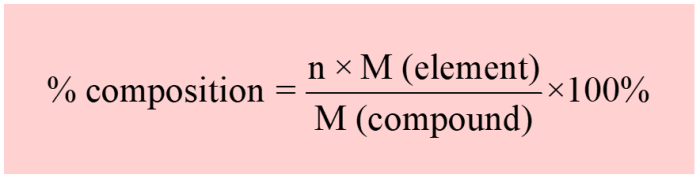How many atoms are in 4.39 g of co2 – How many atoms are in 4.39 g of carbon dioxide (CO2)? This seemingly simple question requires a deeper understanding of molecular composition, molar mass, and Avogadro’s number. Embark on a scientific journey to unravel the atomic realm of CO2 and uncover the intricacies of matter.
Carbon dioxide, a ubiquitous compound in Earth’s atmosphere, plays a crucial role in various biological and chemical processes. Determining the number of atoms in a given mass of CO2 is essential for comprehending its behavior and interactions.
1. Molecular Composition of Carbon Dioxide
Carbon dioxide (CO 2) is a compound composed of carbon and oxygen atoms. It is a colorless, odorless gas that is essential for plant growth and plays a crucial role in the Earth’s atmosphere.
Chemical Formula and Atomic Composition
- Chemical formula: CO 2
- Atomic composition: Each molecule of CO 2contains one carbon atom and two oxygen atoms.
2. Molar Mass and Avogadro’s Number: How Many Atoms Are In 4.39 G Of Co2
Molar Mass
Molar mass is the mass of one mole of a substance, expressed in grams per mole (g/mol). It is used to convert between the mass and the number of moles of a substance.
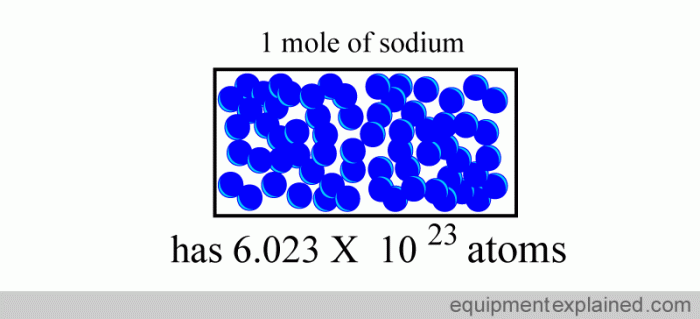
Avogadro’s Number
Avogadro’s number (N A) is the number of atoms or molecules in one mole of a substance. It is equal to 6.022 × 10 23.
Molar Mass of CO2
The molar mass of CO 2is calculated by adding the atomic masses of carbon and oxygen:
Molar mass = (1 × 12.01 g/mol) + (2 × 16.00 g/mol) = 44.01 g/mol
3. Mass to Moles Conversion

To convert a given mass of CO 2to moles, divide the mass by the molar mass:
Moles = Mass (g) / Molar mass (g/mol)
Calculating Moles of CO2
To calculate the number of moles in 4.39 g of CO 2:
Moles = 4.39 g / 44.01 g/mol = 0.0997 mol
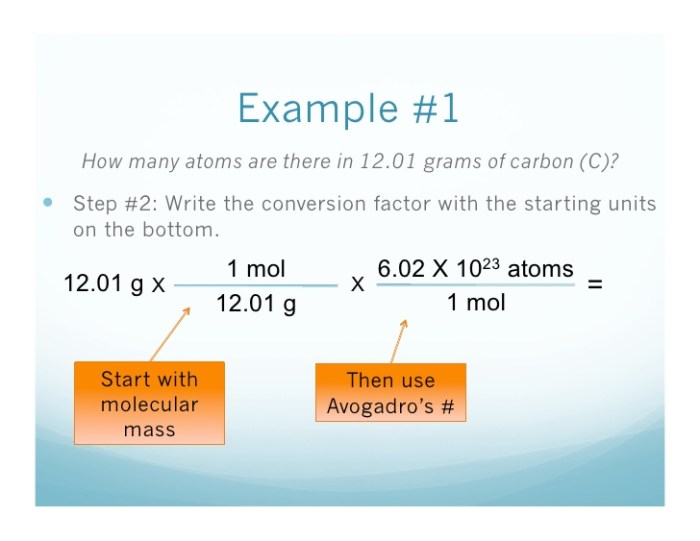
4. Moles to Atoms Conversion
Relationship between Moles and Atoms
The number of atoms in a given number of moles can be calculated using Avogadro’s number:
Atoms = Moles × Avogadro’s number (N A)
Calculating Atoms of CO2, How many atoms are in 4.39 g of co2
To calculate the number of atoms in 4.39 g of CO 2, first convert the mass to moles and then multiply by Avogadro’s number:
Atoms = 0.0997 mol × 6.022 × 10 23atoms/mol = 6.00 × 10 21atoms
Essential Questionnaire
What is the molecular formula for carbon dioxide?
CO2
How many carbon atoms are in a molecule of CO2?
1
How many oxygen atoms are in a molecule of CO2?
2
What is Avogadro’s number?
6.022 x 10^23